
Living with diabetes is a constant balancing act. It’s like doing everything in your daily life while keeping a balloon in the air.
That’s where the #BlueBalloonChallenge comes in! This year, we will focus on raising awareness and shining a light on parents and caregivers who support loved ones living with diabetes.
We’re partnering with Beyond Type 1 and their mission to change what it means to live with diabetes, all to help people with diabetes not just survive, but thrive.
Take part in the challenge and together, we can make the invisible, visible.How to join the challenge and spread awareness
Capture yourself
Record a video or take a photo of yourself (alone or with a parent or caregiver) balancing a balloon during an activity – feel free to get creative!
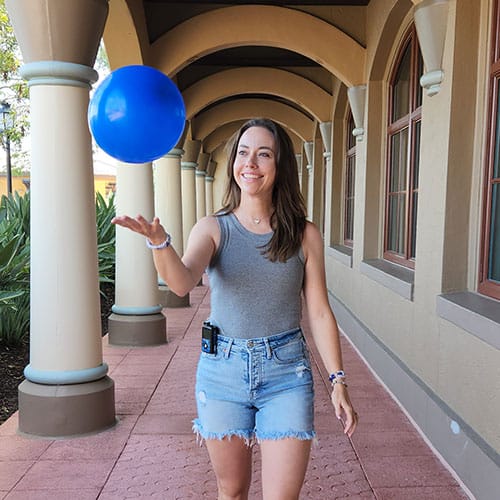
Share it
Tag @medtronicdiabetes and use the hashtag #BlueBalloonChallenge when you share it on social media for a chance to be featured.








