Glossary of symbols
| Symbol | Standard reference | Standard title | Symbol title | Explanatory text |
|---|---|---|---|---|
 |
EN 980, Clause 5.12 | Symbols for use in the labelling of medical devices. | Manufacturer | Indicates the medical device manufacturer. |
| ISO 15223-1, Clause 5.1.1 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied. | |||
| ISO 7000-3082 | Graphical symbols for use on equipment. | |||
 |
ISO 15223-1, Clause 5.1.8 | Medical devices — Symbols to be used with information to be supplied by the manufacturer. | Importer | Indicates the entity importing the medical device into the locale. |
| ISO 7000-3725 | Graphical symbols for use on equipment. | |||
 |
ISO 15223-1, Clause 5.1.9 | Medical devices — Symbols to be used with information to be supplied by the manufacturer. | Distributor | Indicates the entity distributing the medical device into the locale. |
| ISO 7000-3724 | Graphical symbols for use on equipment. | |||
 |
EN 980, Clause 5.6 | Symbols for use in the labelling of medical devices. | Date of manufacture | Indicates the date when the medical device was manufactured. |
| ISO 15223-1, Clause 5.1.3 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied. | |||
| ISO 7000-2497 | Graphical symbols for use on equipment. | |||
 |
EN 60417-6049 | Graphical symbols for use on equipment. | Country of origin | To identify the country of manufacture of products. |
| ISO 3166-1 | Codes for the representation of names of countries and their subdivisions — Part 1:Country codes. | |||
 |
EN 980, Clause 5.13 | Symbols for use in the labelling of medical devices. | Authorized European representative | Indicates the Authorized representative in the European Community/European Union. |
| ISO 15223-1, Clause 5.1.2 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied. | |||
 |
EN 980, Clause 5.10 | Symbols for use in the labelling of medical devices. | Catalogue or model number | Indicates the manufacturer's catalogue number so that the medical device can be identified. |
| ISO 15223-1, Clause 5.1.6 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied. | |||
| ISO 7000- 2493 | Graphical symbols for use on equipment. | |||
 |
ISO 15223-1, Clause 5.1.10 | Medical devices — Symbols to be used with information to be supplied by the manufacturer. | Model Number | Indicates the model number or type number of a product. |
| IEC 60417-6050 | Graphical symbols for use on equipment. | |||
 |
EN 980, Clause 5.5 | Symbols for use in the labelling of medical devices. | Serial number | Indicates the manufacturer's serial number so that a specific medical device can be identified. |
| ISO 15223-1, Clause 5.1.7 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied. | |||
| ISO 7000-2498 | Graphical symbols for use on equipment. | |||
 |
EN 980, Clause 5.4 | Symbols for use in the labelling of medical devices. | Batch code | Indicates the manufacturer's batch code so that the batch or lot can be identified. |
| ISO 15223-1, Clause 5.1.5 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied. | |||
| ISO 7000-2492 | Graphical symbols for use on equipment. | |||
 |
EN 980, Clause 5.3 | Symbols for use in the labelling of medical devices. | Use by | Indicates the date after which the medical device is not to be used. |
| ISO 15223-1, Clause 5.1.4 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied. | |||
| ISO 7000-2607 | Graphical symbols for use on equipment. | |||
 |
EN 980, Clause 5.16 | Symbols for use in the labelling of medical devices. | In vitro diagnostic medical device | Indicates a medical device that is intended to be used as an in vitro diagnostic medical device. |
| ISO 15223-1, Clause 5.5.1 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied. | |||
 NOTE: may be accompanied by www.medtronic.com/manuals |
ISO 7010-M002, IEC 60601-1, Table D.2, Symbol 10 | Medical electrical equipment — Part 1: General requirements for basic safety and essential performance. | Follow instructions for use or follow electronic instructions for use. | Follow instructions for use. |
 NOTE: may be accompanied by www.medtronic.com/manuals |
EN 980, Clause 5.18 | Symbols for use in the labelling of medical devices. | Consult instructions for use or consult electronic instructions for use. | Indicates the need for the user to consult the instructions for use. |
| ISO 15223-1, Clause 5.4.3 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied. | |||
| IEC 60601-1, Table D.1, Symbol 11 | Medical electrical equipment — Part 1: General requirements for basic safety and essential performance. | |||
| ISO 7000-1641 | Graphical symbols for use on equipment. | |||
 |
EN 980, Clause 5.11 | Symbols for use in the labelling of medical devices. | Caution: Read all warnings and precautions in instructions for use | Indicates the need for the user to consult the instructions for use for important cautionary information such as warnings and precautions that cannot, for a variety of reasons, be presented on the medical device itself. |
| ISO 15223-1, Clause 5.4.4 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied. | |||
| IEC 60601-1, Table D.1, Symbol 10 | Medical electrical equipment — Part 1: General requirements for basic safety and essential performance. | |||
| ISO 7000-0434 | Graphical symbols for use on equipment. | |||
 |
IEC 60601-1-2:2007, Clause 5.1.1 | Medical electrical equipment — Part 1-2: General requirements for basic safety and essential performance — Collateral standard: Electromagnetic compatibility — Requirements and tests | Non-ionizing electromagnetic radiation | To indicate generally elevated, potentially hazardous, levels of nonionizing radiation, or to indicate equipment or systems e.g. in the medical electrical area that include RF transmitters or that intentionally apply RF electromagnetic energy for diagnosis or treatment. |
| IEC 60417-5140 | Graphical symbols for use on equipment. | |||
| IEC 60878-5140 | Graphical symbols for electrical equipment in medical practice. | |||
 |
ISO 15223-1, Clause 5.3.8 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied. | Storage humidity range | Indicates the range of humidity to which the medical device can be safely exposed. |
| ISO 7000-2620 | Graphical symbols for use on equipment. | |||
 |
EN 980, Clause 5.17.3 | Symbols for use in the labelling of medical devices. | Storage temperature range | Indicates the temperature limits to which the medical device can be safely exposed. |
| ISO 15223-1, Clause 5.3.7 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied. | |||
| ISO 7000-0632 | Graphical symbols for use on equipment | |||
 |
ISO 15223-1, Clause 5.3.9 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied. | Atmospheric Pressure | Indicates the range of atmospheric pressure to which the medical device can be safely exposed. |
| ISO 7000-2621 | Graphical symbols on equipment | |||
 |
ASTM F2503 | Standard Practice for Marking Medical Devices and Other Items for Safety in the Magnetic Resonance Environment. | Magnetic Resonance (MR) unsafe | Keep away from magnetic resonance imaging (MRI) equipment. |
 |
IEC 60417-5639 | Graphical Symbols for Use on Equipment (derivative symbol and meaning) | Rechargeable battery | Recharge by date |
| ISO 7000-5639 | ||||
| IEC 60417-5546 | Battery check | |||
| ISO 7000-5546 | ||||
 |
ISO 7000-5007 | Graphical Symbols for Use on Equipment (derivative meaning) | ON (power) | Activate by date |
| ISO 7000-5008 | OFF (power) | |||
| ISO 7000-5010 | OFF (power) | |||
| IEC 60417-5007 | ||||
| IEC 60417-5008 | ||||
| IEC 60417-5010 | ||||
 |
ISO 15223-1, Clause 5.3.2 | Medical devices — Symbols to be used with information to be supplied by the manufacturer. | Keep away from sunlight | Indicates a medical device that needs protection from light sources. |
| ISO 7000-0624 | Graphical symbols for use on equipment. | |||
 |
EN 980, Clause 5.21 | Symbols for use in the labelling of medical devices. | Keep dry | Indicates a medical device that needs to be protected from moisture. |
| ISO 15223-1, Clause 5.3.4 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied. | |||
| ISO 7000-0626 | Graphical symbols for use on equipment. | |||
 |
ISO 15223-1, Clause 5.3.1 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied. | Fragile, handle with care | Indicates a medical device that can be broken or damaged if not handled carefully. |
| ISO 7000-0621 | Graphical symbols for use on equipment. | |||
 |
IEC 60601-1, Table D.1, Symbol 20 | Medical electrical equipment — | Type BF applied part | To identify a type BF applied part complying with IEC 60601-1. |
| IEC 60417- 5333 | Part 1: General requirements for basic safety and essential performance. | |||
 |
IEC 60601-1, Table D.1, Symbol 19 | Medical electrical equipment — Part 1: General requirements for basic safety and essential performance. | Type B applied part | To identify a type B applied part complying with IEC 60601-1. |
| IEC 60417-5840 | Graphical symbols for use on equipment. | |||
 |
IEC 60601-1, Table D.3, Symbol 2 IEC 60529 |
Medical electrical equipment — Part 1: General requirements for basic safety and essential performance.
Degrees of Protection Provided by Enclosures (IP Code). |
Degree of Ingress Protection Provided by Enclosure | Manufacturer-determined degree of particle and water ingress protection, where...
N1= degree of protection from particulates (scale of 0-6); and N2 = degree of protection from water (scale of 0-8) NOTE When a characteristic numeral is not required to be specified, it is replaced by the letter "X". |
 |
Protection against insertion of large objects and dripping water. | |||
 |
Protected against solid foreign objects of 12. 5 mm and greater, and against the effects of continuous immersion in water. | |||
 |
Protected against solid foreign objects of 1.0 mm and greater, and against the effects of continuous immersion in water. | |||
 |
Protected against the effects of continuous immersion in water. | |||
 |
Protected against the effects of temporary immersion in water. | |||
 |
ISO 15223-1, Clause 5.4.7 | Medical devices — Symbols to be used with information to be supplied by the manufacturer. | Contains a medicinal substance | Indicates a medical device that contains or incorporates a medicinal substance. |
| ISO 7000-3701 | Graphical symbols for use on equipment. | |||
 |
ISO 15223-1, Clause 5.4.6 | Medical devices — Symbols to be used with information to be supplied by the manufacturer. | Contains human blood or plasma derivatives | Indicates a medical device that contains or incorporates human blood or plasma derivatives. |
| ISO 7000-3701 | Graphical symbols for use on equipment. | |||
 |
ISO 15223-1, Clause 5.4.9 | Medical devices — Symbols to be used with information to be supplied by the manufacturer. | Contains biological material of human origin | Indicates a medical device that contains biological tissue, cells, or their derivatives, of human origin. |
| ISO 7000-3701 | Graphical symbols for use on equipment. | |||
 |
ISO 15223-1, Clause 5.4.10 | Medical devices — Symbols to be used with information to be supplied by the manufacturer. | Contains hazardous substances | Indicates a medical device that contains substances that can be carcinogenic, mutagenic, reprotoxic (CMR), or substances with endocrine disrupting properties. |
| ISO 7000-3723 | Graphical symbols for use on equipment. | |||
 |
ISO 15223-1, Clause 5.4.11 | Medical devices — Symbols to be used with information to be supplied by the manufacturer. | Contains nano materials | Indicates a medical device that contains nano materials. |
| ISO 7000-3703 | Graphical symbols for use on equipment. | |||
 |
ISO 15223-1, Clause 5.4.12 | Medical devices — Symbols to be used with information to be supplied by the manufacturer. | Single patient, multiple use | Indicates a medical device that may be used multiple times (multiple procedures) on a single patient. |
| ISO 7000-3706 | Graphical symbols for use on equipment. | |||
 |
ISO 15223-1, Clause 5.4.1 | Medical devices — Symbols to be used with information to be supplied by the manufacturer. | Biological risks | Indicates that there are potential biological risks associated with the medical device. |
| ISO 7000-0659 | Graphical symbols for use on equipment. | |||
 |
EN 980, Clause 5.2 | Symbols for use in the labelling of medical devices. | Do not reuse | Indicates a medical device that is intended for one use, or for use on a single patient during a single procedure. |
| ISO 15223-1, Clause 5.4.2 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied. | |||
| ISO 7000-1051 | Graphical symbols for use on equipment. | |||
 |
EN 980, Clause 5.22 | Symbols for use in the labelling of medical devices. | Do not resterilize | Indicates a medical device that is not to be resterilized. |
| ISO 15223-1, Clause 5.2.6 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied. | |||
| ISO 7000-2608 | Graphical symbols for use on equipment. | |||
 |
ISO 7000-3079 | Graphical symbols for use on equipment. | Open here | To identify the location where the package can be opened and to indicate the method of opening it. |
 |
EN 980, Clause 6.2 | Symbols for use in the labelling of medical devices. | Contains natural rubber or latex | Indicates the presence of natural rubber or dry natural rubber latex as a material of construction within the medical device or the packaging of a medical device. |
| ISO 15223-1, Clause 5.4.5 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied. | |||
 |
EN 980, Clause 5.23 | Symbols for use in the labelling of medical devices. | Non sterile | Indicates a medical device that has not been subjected to a sterilization process. |
| ISO 15223-1, Clause 5.2.7 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied. | |||
| ISO 7000-2609 | Graphical symbols for use on equipment. | |||
 |
EN 980, Clause 5.7 | Symbols for use in the labelling of medical devices. | Product subjected to a sterilization process | Indicates a medical device that has been subjected to a sterilization process. |
| ISO 15223-1, Clause 5.2.1 | Medical devices — Symbols to be used with medical device labels, labelling and information... | |||
| ISO 7000-2499 | Graphical symbols for use on Equipment. | |||
 |
EN 980, Clause 5.8.2 | Symbols for use in the labelling of medical devices. | Sterilized by ethylene oxide treatment | Indicates a medical device that has been sterilized using ethylene oxide. |
| ISO 15223-1, Clause 5.2.3 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied. | |||
| ISO 7000-2501 | Graphical symbols for use on equipment. | |||
 |
EN 980, Clause 5.8.3 | Symbols for use in the labelling of medical devices. | Sterilized using irradiation | Indicates a medical device that has been sterilized using irradiation. |
| ISO 15223-1, Clause 5.2.4 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied. | |||
| ISO 7000-2502 | Graphical symbols for use on equipment. | |||
 |
EN 980, Clause 5.8.4 | Symbols for use in the labelling of medical devices. | Sterilized using steam or dry heat | Indicates a medical device that has been sterilized using steam or dry heat. |
| ISO 15223-1, Clause 5.2.5 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied. | |||
| ISO 7000-2503 | Graphical symbols for use on equipment. | |||
 |
ISO 7000-3707 | Graphical symbols for use on equipment | Single sterile barrier system | Indicates a single sterile barrier system |
| ISO 15223-1, Clause 5.2.11 | Medical devices — Symbols to be used with information to be supplied by the manufacturer. | |||
 |
ISO 15223-1, Clause 5.6.3 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied . | Non-pyrogenic | Indicates a medical device that is non-pyrogenic. |
| ISO 7000-2724 | Graphical symbols for use on equipment. | |||
 |
ISO 15223-1:2012 (Annex B, 15223-1:2012) | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied | Non-pyrogenic | Indicates a medical device that is non-pyrogenic. |
 |
ISO 15223-1:2012 (Annex B, 15223-1:2012) | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied | Not made with natural rubber latex | Not made with natural rubber latex. |
 |
ISO 15223-1, Clause 5.2.8 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied . | Do not use if package is damaged and consult instructions for use. | Indicates a medical device that should not be used if the package has been damaged or opened. |
| ISO 7000-2606 | Graphical symbols for use on equipment. | |||
 |
EN 50419 | Marking of Electrical and Electronic Equipment in accordance with Article 11(2) of Directive 2002/96/EC (WEEE). | Recycle: Electronic Equipment | Do not dispose of this product in unsorted municipal waste stream. |
|
|
EN 15986, Clause 4.2 | Symbol for use in the labelling of medical devices. Requirements for labelling of medical devices containing phthalates. | DEHP di(2-ethylexyl) phthalate BBP (Benzyl butyl phthalate) DBP (Dibutyl phthalate) |
Contains phthalate |
 |
ISO 15223 1 Clause 5.7.1 | Medical devices — Symbols to be used with information to be supplied by the manufacturer. | Patient number | Indicates a unique number associated with an individual patient. |
| ISO 7000-2610 | Graphical symbols for use on equipment. | |||
 |
ISO 15223 1 Clause 5.7.2 | Medical devices — Symbols to be used with information to be supplied by the manufacturer. | Patient name | Indicates the name of the patient. |
| ISO 7000-3726 | Graphical symbols for use on equipment. | |||
 |
ISO 15223 1 Clause 5.7.3 | Medical devices — Symbols to be used with information to be supplied by the manufacturer. | Patient identification | Indicates the identification data of the patient. |
| IEC 60417-5664 | Graphical symbols for use on equipment. | |||
 |
ISO 15223 1 Clause 5.7.4 | Medical devices — Symbols to be used with information to be supplied by the manufacturer. | Patient information website | Indicates a website where a patient can obtain additional information on the medical product. |
| ISO 7000-3705 | Graphical symbols for use on equipment. | |||
 |
ISO 15223 1 Clause 5.7.5 | Medical devices — Symbols to be used with information to be supplied by the manufacturer. | Health care centre or doctor | Indicates the address of the health care centre or doctor where medical information about the patient may be found. |
| ISO 7001 PI PF 044 | Public information symbols. | |||
 |
ISO 15223 1 Clause 5.7.6 | Medical devices — Symbols to be used with information to be supplied by the manufacturer. | Date | Indicates the date that information was entered or a medical procedure took place. |
| IEC 60417-5662 | Graphical symbols for use on equipment. | |||
 |
ISO/DIS 15223- 1:2020(E) DRAFT Reference no. 5.7.7 | Medical Devices — Symbols to be used with medical device labels, labeling, and information to be supplied — Part 1: General requirements. | Medical device | Indicates the item is a medical device. |
 |
ISO 15223 1 Clause 5.7.8 | Medical devices — Symbols to be used with information to be supplied by the manufacturer. | Translation | Indicates that the original medical device information has undergone a translation which supplements or replaces the original information. |
| ISO 7000-3728 | Graphical symbols for use on equipment. | |||
 |
ISO 15223 1 Clause 5.7.9 | Medical devices — Symbols to be used with information to be supplied by the manufacturer. | Repackaging | Indicates that a modification to the original medical device packaging configuration has occurred. |
| ISO 7000-3727 | Graphical symbols for use on equipment. | |||
 |
ISO 15223 1 Clause 5.7.10 | Medical devices — Symbols to be used with information to be supplied by the manufacturer. | Unique device identifier | Indicates a carrier that contains unique device identifier information. |
Symbols not from standards
| Symbol | Reference | Title | Symbol title | Explanatory text |
|---|---|---|---|---|
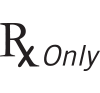 |
21 CFR 801.15(c)(1)(i)F | Labeling-Medical devices; prominence of required label statements. | Prescription only | Requires prescription in the United States. |
| 21 CFR 801.109 | Labeling-Prescription devices. | |||
 |
765/2008/EC 768/2008/EC MDD 93/42/EEC Articles 4,11,12,17, Annex II ) RED 2014/53/EU (Articles 19, 20, Annex II) |
Conformité Européenne (European Conformity). This symbol means that the device fully complies with applicable European Union Acts. | CE marking | Signifies European technical conformity. |
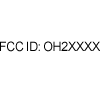 |
(47 CFR Part 2 & 15) | Code of Federal Regulations Title 47: Telecommunication Part 2:Frequency allocations and radio treaty matters; general rules and regulations Part 15: Radio Frequency Devices (US). |
Federal Communication Commission Identifier (FCC ID #) | Complies with United States Regulations for Radio Frequency Devices. |
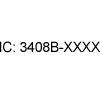 |
(RSS-GEN and RSP-100) | Radio Standards Specifications (CA). | Innovation, Science and Economic Development (ISED) Canada Identifier (IC:#) | Complies with ISED Canada Radio Standards Specifications. |
 |
Radiocommunications (Compliance labelling- Devices) Notice and Radiocommunications Labelling (Electromagnetic Compatibility) Notice | Australia/New Zealand (ANZ) Radiocommunications Requirements. | Regulatory Compliance Mark (RCM) | Complies with ANZ radiocommunications requirements. |
 |
Article 38 and Notice 88 | Japan Radio Law and Ordinance Concerning Technical regulations Conformity Certification of Specified Radio Equipment. | Giteki; Technical Conformity Mark | Complies with Japan Radio Law. |
 |
(Trademarks of Bluetooth Special Interest Group (SIG) | N/A | Bluetooth® wireless or enabled technology | Bluetooth® wireless or enabled technology. |
 |
N/A | N/A | Configuration | Configuration or unique version identifier. |
 |
N/A | N/A | Manufacturing Site | Indicates the manufacturing site of the device. A manufacturing site is the facility where the product is produced, transformed, or assembled into a medical device. |
 |
N/A | N/A | Replace every three days | Indicates the user to replace on 3rd day of use (or as directed by physician). |

or
 |
N/A | N/A | Replace every [specify] days | Indicates the user to replace as specified (or as directed by physician). |
 |
N/A | N/A | Global Trade Identification Number | Custom symbol denoting global trade item number. |
 |
N/A | N/A | Reimbursement number | Custom symbol |
The Bluetooth® word mark and logos are registered trademarks owned by Bluetooth SIG, Inc. and any use of such marks by Medtronic is under license.


