Insulin pump therapy for type 2 diabetes.
When a person is newly diagnosed with type 2 diabetes, their healthcare professionals may offer various treatment options such as oral medication, insulin shots, or insulin pump therapy. People living with type 2 diabetes may choose insulin pump therapy as it requires fewer insulin injections or insulin shots.*
What are the advantages of insulin pump therapy over daily injections for type 2 diabetes?
Studies have shown that A1C reduction can significantly reduce the occurrence of long-term complications.1,2 With insulin pump therapy, you can worry less about your risk for long-term complications, such as:





Insulin pump therapy may reduce some of the hassles associated with other therapy options:
- Syringe or insulin pen
- Oral medication
- Using more insulin
Insulin pump therapy is clinically proven to reduce A1C better than multiple daily insulin shots for people living with type 2 diabetes.3



Insulin pump therapy. Live more. Worry less.
Instead of using long-acting insulin (like Lantus or Levemir), which can pool under your skin or not absorb evenly into your body, the insulin pump continuously delivers only rapid-acting insulin (like Humalog or NovoLog), which is absorbed more predictably.4 Studies show you’re up to six times more likely to achieve your target A1C with continuous insulin delivery than with shots.5
With insulin pump therapy, you can eat when you want and go on outings without worrying about injections. If you want to eat, just push a couple of buttons on the pump to get more insulin. If you want to delay a meal, you can.

Multiple shots per day can mean more than 120 shots per month. With insulin pump therapy, you only have to insert your infusion set 10 to 12 times per month.
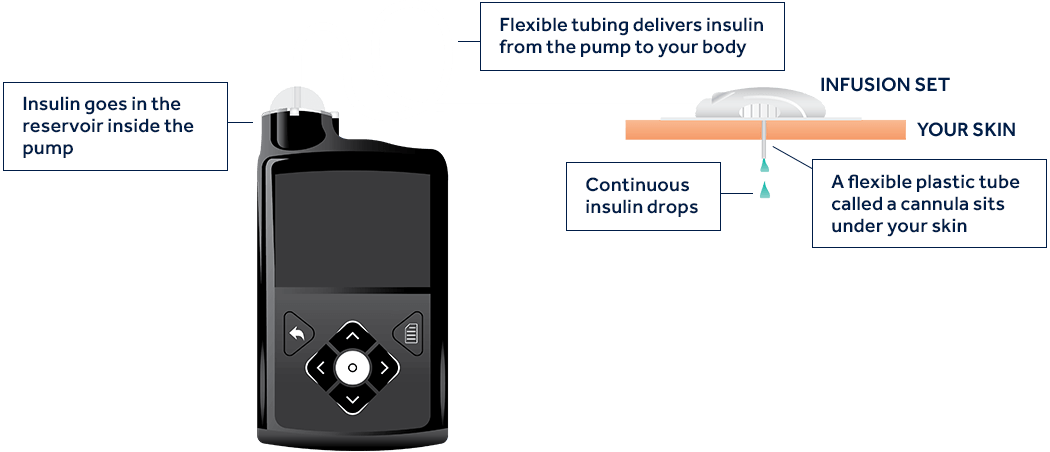
How does an insulin pump work?
An insulin pump is a small device that mimics some of the ways a healthy pancreas works. It delivers continuous and customized doses of rapid-acting insulin 24 hours a day to match your body’s needs.
Who can benefit from insulin pump therapy?
You may be a candidate for insulin pump therapy if you:
- Are taking insulin injections
- Have an A1C greater than 7%
- Forget to take your insulin injections
- Have frequent high or low blood sugars
- Enjoy an active lifestyle
- Desire to spend less time managing your diabetes
Why wait? Get started on insulin pump therapy today.
References
* Assumes 4 injections per day for 30 days and one infusion set change every two to three days.
1 Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
2 U.K. Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complication in patients with type 2 diabetes (UKPDS 33). The Lancet. 1998;352:837-853.
3 Reznik Y, Cohen O, Aronson R, et al, Insulin pump treatment compared with multiple daily injections for treatment of type 2 diabetes (OpT2mise): a randomized open-label controlled trial. The Lancet. 2014; 2014;384:1265-1272.
4 Lauritzen T, Pramming S, Deckert T, Binder C, Pharmacokinetics of continuous subcutaneous insulin infusion. Diabetologia. 1983;24(5):326-329.
5 Doyle EA, Weinzimer, Steffen AT, et al. A randomized, prospective trial comparing the efficacy of continuous subcutaneous insulin infusion with multiple daily injections using insulin glargine. Diabetes Care. 2014;27(5)1554-1558.
Advertisements
Aronson R, Reznik Y, Conget I, et al. Sustained efficacy of insulin pump therapy compared with multiple daily injections in type 2 diabetes: 12 month data from the OpT2mise randomized trial. Diabetes Obesity and Metabolism. 2016;18:500–507.
Wolff-McDonagh, P, Kaufmann J, Foreman S, WisotskyS, WisotskyJ, Wexler C. Using insulin pump therapy in poorly controlled type 2 diabetes. The Diabetes Educator. 2010;36:657-665.
dQ&A Diabetes Connections Q4 2019 surveyed market share data, n = 172.
Customers who take part in the evaluation program will only be billed following the 6-week evaluation period if keeping the device. Insurance will be billed as normal and refunded if participant chooses not to keep the device and complies with program requirements. Offer only available to new type 2 diabetes MiniMed™ 630G system customers. See program agreement for more details. Program terms subject to change at any time and without notice.
Assumes four injections per day for 30 days and one infusion set change every two to three days.
Important Safety Information: MiniMed™ 780G system with SmartGuard™ technology with Instinct sensor, Simplera Sync™ sensor, and Guardian™ 4 sensor
The MiniMed™ 780G system is intended for the continuous delivery of basal insulin at selectable rates and the administration of insulin boluses at selectable rates for the management of type 1 diabetes mellitus in persons 7 years of age and older, and of type 2 diabetes mellitus in persons 18 years of age and older requiring insulin. The system is also intended to continuously monitor glucose vales in the fluid under the skin.
The MiniMed™ 780G System includes SmartGuard™ technology, which can be programmed to automatically adjust insulin delivery based on the continuous glucose monitoring (CGM) sensor glucose values and can suspend delivery of insulin when the sensor glucose (SG) value falls below or is predicted to fall below predefined threshold values. The system is intended for use with connected sensors, including the Simplera Sync™ and Guardian™ 4 sensors and integrated continuous glucose monitors, including the Instinct sensor, each of which has different wear-time, form factor, insertion site, and other distinguishing characteristics that relate to sensor performance. Consult the appropriate sensor user guide when using the system. Discuss treatment decisions with your HCP.
WARNING: Do not use the SmartGuard™ feature for people who require less than 8 units or more than 250 units of total daily insulin per day. A total daily dose of at least 8 units, but no more than 250 units, is required to operate in the SmartGuard™ feature.
WARNING: Do not use the MiniMed™ 780G system until appropriate training has been received from a healthcare professional. Training is essential to ensure the safe use of the MiniMed™ 780G system.
WARNING: Do not use SG values to make treatment decisions, including delivering a bolus, while the pump is in Manual Mode. When the SmartGuard™ feature is active and you are no longer in Manual Mode, the pump uses an SG value, when available, to calculate a bolus amount. However, if your symptoms do not match the SG value, use a blood glucose (BG) meter to confirm the SG value. Failure to confirm glucose levels when your symptoms do not match the SG value can result in the infusion of too much or too little insulin, which may cause hypoglycemia or hyperglycemia.
Pump therapy is not recommended for people whose vision or hearing does not allow for the recognition of pump signals, alerts, or alarms. The safety of the MiniMed™ 780G system has not been studied in pregnant women or in persons using other anti-hyperglycemic therapies that do not include insulin. For complete details of the system, including accessories and additional important safety information concerning indications, contraindications, warnings and precautions associated with the system and its components, please consult https://www.medtronicdiabetes.com/important-safety-information and the appropriate user guides at https://www.medtronicdiabetes.com/download-library.
