Terapia con bomba de insulina. Una opción avanzada para el tratamiento de la diabetes.
Obtenga más información sobre cómo funciona la terapia con bomba de insulina, cuáles son sus beneficios y para qué personas es adecuada.
¿Qué es la terapia con bomba de insulina?
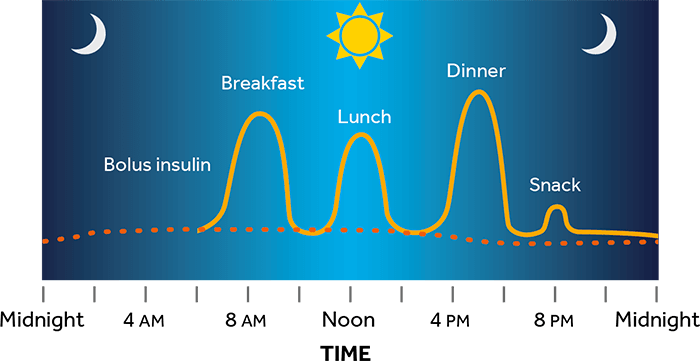
Una bomba de insulina es un pequeño dispositivo que puede ayudarlo a tratar la diabetes. Administra dosis continuas y personalizadas de insulina de acción rápida las 24 horas del día para satisfacer las necesidades del cuerpo. La bomba administra insulina en el cuerpo de dos maneras:
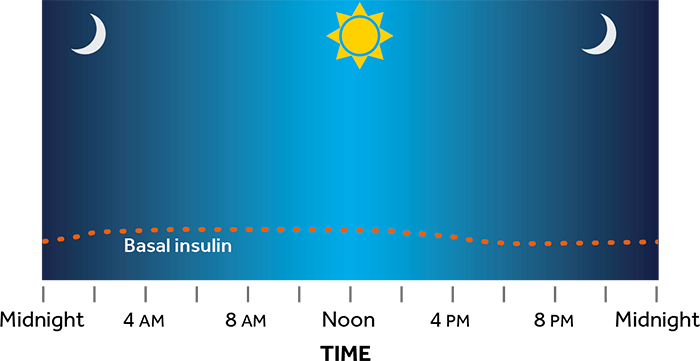
¿Cómo funciona la bomba de insulina?
La bomba administra insulina en el cuerpo a través de un tubo delgado y flexible denominado equipo de infusión.
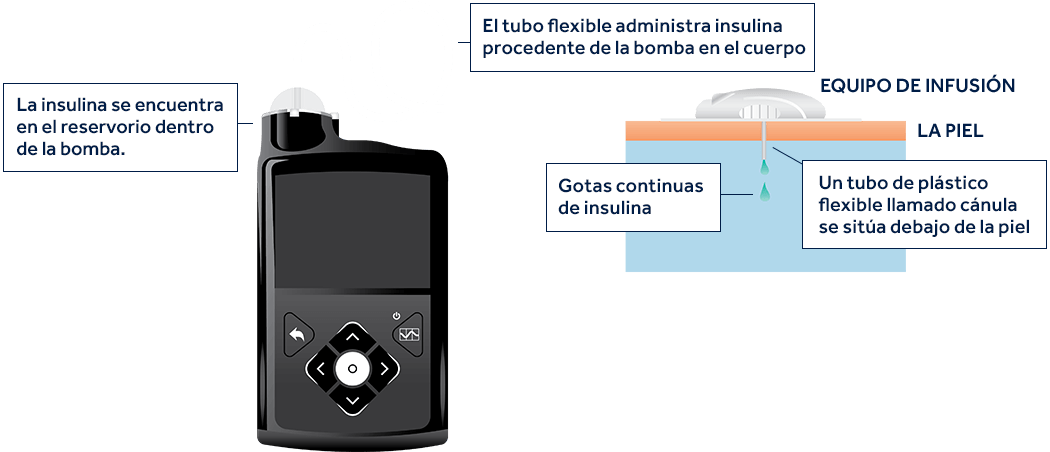
¿Qué componentes se utilizan como parte del sistema de terapia con bomba de insulina?
Varias piezas funcionan en conjunto para administrar dosis continuas de insulina.
Desplácese sobre los componentes que aparecen a continuación para obtener más información.

¿Cuáles son los beneficios de la terapia con bomba de insulina?
Las personas pueden experimentar muchos cambios positivos en sus vidas cuando pasan a la terapia con bomba.
Con la bomba de insulina, solo deberá insertar un equipo de infusión cada pocos días. En el caso de las múltiples inyecciones diarias, las personas suelen inyectarse varias veces al día.
- La bomba de insulina contiene hasta 300 mL de insulina, de modo que no debe preocuparse por transportar todos los suministros necesarios para las inyecciones.
- La insulina se puede administrar de forma fácil y discreta con solo oprimir unos botones.
- La bomba se puede programar para que administre insulina basal a tasas diferentes durante todo el día.
- Puede cambiar la insulina a la hora de las comidas según los alimentos que elija comer.
La calculadora de bolos elimina los cálculos complejos y realiza un seguimiento de la insulina activa. El seguimiento de la insulina activa ayuda a evitar la acumulación de insulina y los niveles bajos.
Puede ayudarlo a lograr mejor control de la glucosa sin tantos altibajos.†
- La bomba de insulina administra insulina de acción rápida que minimiza la variabilidad de la insulina. 1,2
- Administra insulina basal y en bolo para ajustarse mejor a las necesidades de insulina de cada persona.
¿Quién puede usar una bomba de insulina?
Consulte a un profesional de atención médica sobre la terapia con bomba de insulina si usted reúne los siguientes requisitos:
- Recibe tres o más inyecciones de insulina por día.
- Recibe otros medicamentos además de la insulina para controlar la diabetes.
- Quisiera mejorar el tratamiento de la diabetes.
¿Dónde puede usar la bomba de insulina?
La bomba de insulina se puede llevar de muchas formas diferentes. Se puede usar cómodamente durante el trabajo, la actividad física, las ocasiones formales y la vida diaria. Desplácese sobre las imágenes que aparecen a continuación para descubrir cómo otras personas usan su bomba.
¿Cubre el seguro la bomba de insulina?
Cuando se habla del tratamiento de la diabetes, el costo no debería ser un obstáculo para acceder a tecnología avanzada para la diabetes. Nuestro equipo trabajará a su lado para que pueda experimentar los beneficios de la terapia con bomba de insulina.
Seguro privado‡
La mayor parte de las compañías de seguro privadas cubren las bombas de insulina en la sección de equipos médicos duraderos de su póliza. Según su cobertura de seguro, es posible que deba pagar un deducible o un porcentaje del costo (coseguro). Si ha alcanzado el deducible y el desembolso máximo, su seguro podría cubrir la bomba de insulina en un 100%.
Seguro del gobierno‡
Los seguros gubernamentales, tales como Medicare y Medicaid, pueden cubrir las bombas de insulina en función del estado y otros requisitos. El costo de desembolso de un paciente que posee un seguro del gobierno varía según la póliza.
Procesamiento del seguro
Cuando inicia el proceso para obtener una bomba de insulina, no debe preocuparse por la documentación. Medtronic lo ayudará en cada paso del proceso verificando su seguro, proporcionando un costo estimado de desembolso, recopilando sus documentos y los de su médico, y enviando los documentos requeridos a su compañía de seguro.
Opciones de pago
Si no tiene seguro o necesita ayuda con el costo de desembolso, Medtronic ofrece opciones flexibles de pago y un programa de asistencia financiera para los clientes que reúnen las condiciones.
Frequently asked questions
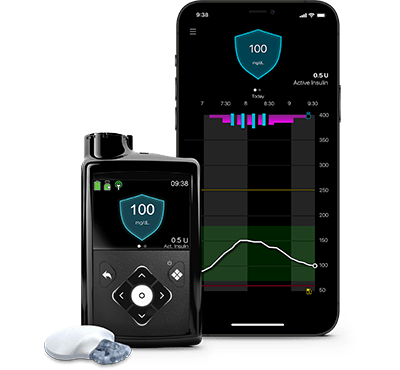
Sistema MiniMed™ 780G
El único sistema con tecnología Meal Detection™** que ajusta y corrige automáticamente† los niveles de glucosa cada 5 minutos.§
* En el momento de la fabricación y cuando el reservorio y el tubo están bien insertados, la bomba es impermeable. Está protegida de los efectos de una inmersión en agua a una profundidad hasta de 3,6 metros (12 pies) durante un máximo de 24 horas. Está clasificada como IPX8. Para obtener más detalles, consulte la guía del usuario. El sensor y el transmisor son resistentes al agua a una profundidad de 2,4 metros (8 pies) durante un máximo de 30 minutos. Es posible que las lecturas del MCG no se transmitan desde el MCG a la bomba mientras se encuentra en el agua.
‡ Los lineamientos de cobertura para las bombas de insulina varían y están sujetos a las pólizas médicas publicadas por las compañías de seguro en vigencia al momento en que se solicitan los servicios.
**Taking a bolus 15 – 20 min before a meal provides significant improvement in post meal control. We recommend taking a meal bolus.
† Refers to auto correct, which provides bolus assistance. Can deliver all correction doses automatically without user interaction, feature can be turned on and off.
§ Refers to SmartGuard™ feature. Some user interaction required. Individual results may vary.
Φ Optional CGM
1. Heinemann L, Weyer C, Rauhaus M, Heinrichs S, Heise T. Variability of the metabolic effect of soluble insulin and the rapid-acting insulin anolog insulin aspart.Diabetes Care.1998;21:1910-1914.
2. Heise T, Nosek L, Ronn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53:1614-1620.
3. Bergenstal RM,Tamborlane WV, Ahmann A, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363:311–320.
Las personas fotografiadas y citadas en esta sección recibieron una compensación por el tiempo dedicado y por permitirnos fotografiarlas a ellas y sus familias. Los pensamientos y las opiniones de estas personas son propios.
Información de seguridad importante: sistema MiniMed™ 780G con tecnología SmartGuard™ con sensor Guardian™ 4
El sistema MiniMed™ 780G tiene como fin la administración continua de insulina basal a índices seleccionables y la administración de bolus de insulina en cantidades seleccionables para el tratamiento de la diabetes mellitus d tipo 1 en personas de siete años en adelante que requieran insulina, así como también para el control continuo y el análisis de tendencias de los niveles de glucosa en el líquido debajo de la piel. El Sistema MiniMed™ 780G incluye la tecnología SmartGuard™, que se puede programar para ajustar de manera automática la administración de insulina según los valores de glucosa registrados por el sensor en la monitorización continua de glucosa (MCG) y puede suspender la administración de insulina cuando el valor de glucosa del sensor (SG) cae por debajo o se prevé que caerá por debajo de los valores límites predefinidos.
El sistema MiniMed™ 780G de Medtronic consta de los siguientes dispositivos: bomba de insulina MiniMed™ 780G, transmisor Guardian™ 4, sensor Guardian™ 4, dispositivo de inserción One-press, medidor de glucosa en sangre Accu-Chek™ Guide Link y tiras reactivas Accu-Chek™ Guide. El sistema requiere la prescripción de un profesional de atención médica.
El sensor Guardian™ 4 está indicado para utilizarse con el sistema MiniMed™ 780G y con el transmisor Guardian 4 para controlar los niveles de glucosa en el tratamiento de la diabetes. El sensor está diseñado para un solo uso y requiere la prescripción de un profesional de atención médica. El sensor Guardian™ 4 está indicado para utilizarse de manera continuada durante siete días como máximo.
El sensor Guardian™ 4 no está indicado para hacer directamente ajustes en el tratamiento mientras el MiniMed™780G funciona en modo manual. Todos los ajustes de tratamiento deben basarse en mediciones obtenidas con un medidor de glucosa en la sangre y no en los valores que brinde el sensor Guardian™ 4. El sensor Guardian™ 4 ha sido evaluado y cuenta con la aprobación para su uso en pacientes a partir de los 7 años y únicamente en la zona de inserción del brazo. No utilice el sensor Guardian™ 4 en el abdomen ni en ningún otro lugar del cuerpo, ni siquiera los glúteos, ya que un funcionamiento desconocido o diferente podría provocar hipoglucemia o hiperglucemia.
ADVERTENCIA: No utilice la función SmartGuard™ en personas que necesitan una dosis de insulina diaria total inferior a 8 unidades o superior a 250 unidades. Se requiere una dosis diaria total de al menos 8 unidades, pero no más de 250 unidades, para operar en la función SmartGuard™.
ADVERTENCIA: No utilice el sistema MiniMed™ 780G hasta que haya recibido la capacitación adecuada de un profesional de la salud. La capacitación es clave para garantizar el uso seguro del sistema MiniMed™ 780G.
ADVERTENCIA: No utilice los valores de SG para tomar decisiones sobre el tratamiento, como la administración de un bolus, cuando la bomba esté en modo manual. Cuando la función SmartGuard™ está activa y usted ya no se encuentra en el modo manual, la bomba utiliza un valor de SG, cuando está disponible, para calcular una cantidad de bolus. No obstante, si sus síntomas no coinciden con el valor de SG, utilice un medidor de GS para confirmar el valor de SG. Si no se confirman los niveles de glucosa cuando sus síntomas no coinciden con el valor de SG, es posible que se administre una cantidad de insulina excesiva o insuficiente, lo que puede causar hipoglucemia o hiperglucemia.
El tratamiento con bomba no es recomendable para personas cuya capacidad visual o auditiva no les permita reconocer las señales, alertas o alarmas de la bomba. No se ha estudiado la seguridad del sistema MiniMed™ 780G en mujeres embarazadas, en personas con diabetes de tipo 2 ni en personas que están recibiendo otros tratamientos antihiperglucémicos que no incluyen insulina. Para obtener los detalles completos del sistema, que incluye información del producto e información de seguridad importante, como indicaciones, contraindicaciones, advertencias y precauciones en relación con el sistema y sus componentes, consulte https://www.medtronicdiabetes.com/important-safety-information#minimed-780g y la guía del usuario correspondiente en https://www.medtronicdiabetes.com/download-library.
 Ver el video
Ver el video