Continuous glucose monitors for better diabetes management
What are continuous glucose monitors (CGM)?
A CGM is a small device that tracks glucose levels, every few minutes, through a tiny sensor inserted under your skin. Continuous glucose monitoring gives people with diabetes a more complete picture of their glucose levels, which can lead to better lifestyle decisions and better glucose control.
How a CGM system works
A CGM system uses a small, flexible sensor inserted under the skin to measure glucose in the fluid surrounding your cells (called interstitial fluid). The sensor continuously sends glucose readings to a mobile app or insulin pump. Unlike traditional blood glucose tests from a fingerstick, which measure the glucose in your bloodstream, CGMs track glucose levels in real time.
Components
A continuous glucose monitor is typically made up of two main parts. However, previous generation CGMs may also have a third: a separate transmitter.
- The sensor is inserted under the skin and measures glucose levels in the interstitial fluid. Sensors are applied to the body by using either a disposable inserter/applicator or a separate reusable applicator.
- A mobile app or separate receiver displays the real-time glucose readings transmitted from the sensor, as well as trends and alerts, allowing users to monitor their levels conveniently.
- The transmitter (older CGM generations) attaches to the sensor and wirelessly sends glucose readings to a display device. It acts as a bridge between the sensor and the receiver.
Benefits of CGM
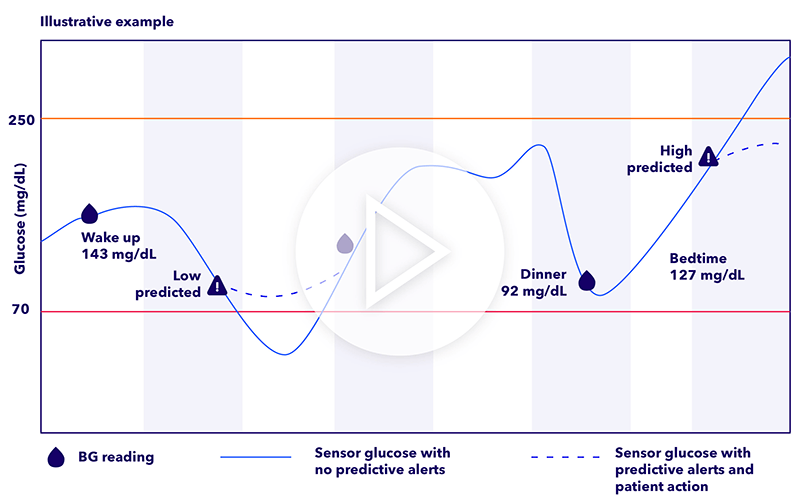
A CGM system gives you a clearer picture of your glucose trends, providing valuable information at regular intervals, including at important times such as prior to driving, before and after exercise, or even overnight. Some of the benefits for using one include:
- Improved glucose control — Research has shown that people with type 1 diabetes who use a CGM experience fewer highs and lows.1
- Personalized diabetes management — By tracking your glucose levels in real time, you get valuable insights that can help you and your healthcare team create a personalized plan to manage your diabetes.2
- Reduced risk of hypoglycemia — The American Diabetes Association recommends CGM as a tool for helping to reduce the risk of hypoglycemia in people with type 1 diabetes.3,4
The CGM device as part of a system
While they provide real-time insights into how your glucose levels are throughout the day, CGMs alone are not enough to effectively manage diabetes. By connecting a CGM to an automated insulin pump or smart insulin pen, you allow technology to help do more of the thinking, remembering, and acting for you while helping improve your Time in Range.
How a CGM works with an insulin pump
The sensor on your body continuously tracks your glucose levels and sends that info wirelessly to your insulin pump and mobile app. An automated insulin delivery system, like the MiniMed™ 780G system, uses the sensor glucose data from the CGM to automatically adjust insulin. From the mobile app, you can see graphs and trends, including actions your insulin pump takes in response to the information.

Compare Medtronic CGM options
Medtronic Diabetes offers a fully integrated automated insulin delivery system that works with the Instinct sensor, made by Abbott, the Simplera Sync™ sensor, Guardian™ 4 sensor, and a smart insulin pen system that’s compatible with the Simplera™ sensor. See all these sensor options side-by-side:
| Instinct sensor, made by Abbott |
Simplera Sync™ sensor | Guardian™ 4 sensor | Simplera™ CGM | |||||
 |
 |
 |
 |
|||||
| Product compatibility | ||||||||
| Product compatibility | MiniMed™ 780G system | MiniMed™ 780G system | MiniMed™ 780G system | InPen™ smart insulin pen system | ||||
| CGM size | ||||||||
| CGM size | 0.11 in height 0.83 in width |
0.19 in height 1.13 in width |
2.0 in height 2.6 in width |
0.19 in height 1.13 in width |
||||
| Wear time | ||||||||
| Wear time | 15 days | Up to 6 days, with 24-hour grace period | Up to 7 days | Up to 6 days, with 24-hour grace period | ||||
Frequently asked questions
People living with diabetes can use a continuous glucose monitor. Insulin-dependent individuals can combine a CGM with their insulin pump or smart insulin pen for a fully integrated system that can help make diabetes management easier.
Yes, you may still need a meter for confirmatory tests, especially if your symptoms don't match your CGM reading. However, some CGMs, like the Instinct sensor, are factory-calibrated and may reduce the number of fingersticks necessary.†,‡
Sensor lifespan depends on brand/model. For instance, the Simplera Sync™ sensor last for up to 6 days with a 24-hour grace period, while the Instinct sensor lasts 15 days.
Yes, a prescription is required to use a CGM. Insurance plans often require prior authorization, and some coverage rules apply.
Most sensors are water-resistant and can be worn while bathing, showering or swimming. The Instinct sensor is water-resistant for up to 3 feet of water for 30 minutes, while Simplera Sync is water-resistantImportant Safety Information: InPen smart insulin pen system.
The InPen system is a reusable insulin pen for people living with diabetes. It can be used to deliver insulin, help calculate insulin doses, and estimate carbohydrates for meals. Those under the age of 7 should only use the device with an adult’s supervision. A healthcare provider must prescribe InPen, provide dosage settings, and discuss all potential benefits and risks. Using the device with incorrect therapy settings may lead to severe highs and lows. The InPen should not be used by those unable to test blood glucose levels or the visually impaired. For additional product and important safety information, click here. for up to 8 feet of water for 30 minutes. Bluetooth connectivity may be impacted when the CGM is underwater and CGM data may not transmit to the pump when immersed.
Talk to a Diabetes Therapy Consultant today!
We will get started on determining your insurance or pharmacy coverage. A member of our team will reach out to you for additional information. If you have questions, please contact us at 888-882-8602 (M–F, 9 a.m.–6 p.m. CT).
Footnotes
† Fingersticks required in manual mode & to enter SmartGuard™. If symptoms don’t match alerts & readings, use a fingerstick. Refer to user guide. Pivotal trial participants spend average of >93% in SmartGuard™.
‡ Use a blood glucose (BG) meter reading to make treatment decisions when you see
the Check BG icon during the first 12 hours of wearing a sensor. Do not use sensor glucose (SG) readings to make treatment decisions during the first 12 hours of wearing a sensor.
References
1. Battelino et al. (2019). The effect of continuous glucose monitoring on glucose control in patients with type 1 diabetes: a systematic review and meta-analysis. Journal of Clinical Endocrinology and Metabolism, 104(11), 5320-5328.
2. Riddle et al. (2020). The role of continuous glucose monitoring in diabetes management. Journal of Diabetes Research, 2020, 1-9.
3. American Diabetes Association. (2022). Standards of medical care in diabetes-2022. Diabetes Care, 45(Supplement 1), S1-S212.
4. Le MQ et al. (2020). Detection of hypoglycemia using a continuous glucose monitoring system in patients with type 1 diabetes. Diabetes Technology & Therapeutics, 22(3), 238-244.
©2025 Medtronic. MiniMed and MiniMed logo are trademarks of Medtronic MiniMed, Inc. The sensor shape and appearance, Abbott, and “a” logo are marks and/or designs of the Abbott group of companies in various territories and used under license. Sensor image ©2025 Abbott. ™*Third–party brands are trademarks of their respective owners.
Important Safety Information: MiniMed™ 780G system with SmartGuard™ technology with Instinct sensor, Simplera Sync™ sensor, and Guardian™ 4 sensor
The MiniMed™ 780G system is intended for the continuous delivery of basal insulin at selectable rates and the administration of insulin boluses at selectable rates for the management of type 1 diabetes mellitus in persons 7 years of age and older using the Instinct, Simplera Sync™ or Guardian™ 4 sensor, and of type 2 diabetes mellitus in persons 18 years of age and older requiring insulin using the Simplera Sync™ or Guardian™ 4 sensor.
The MiniMed™ 780G System includes SmartGuard™ technology, which can be programmed to automatically adjust insulin delivery based on the continuous glucose monitoring (CGM) sensor glucose values and can suspend delivery of insulin when the sensor glucose (SG) value falls below or is predicted to fall below predefined threshold values. The system is intended for use with connected sensors, including the Simplera Sync™ and Guardian™ 4 sensors and integrated continuous glucose monitors, including the Instinct sensor, each of which has different wear-time, form factor, insertion site, and other distinguishing characteristics that relate to sensor performance. Consult the appropriate sensor user guide when using the system. Discuss treatment decisions with your HCP.
WARNING: Do not use the SmartGuard™ feature for people who require less than 8 units or more than 250 units of total daily insulin per day. A total daily dose of at least 8 units, but no more than 250 units, is required to operate in the SmartGuard™ feature.
WARNING: Do not use the MiniMed™ 780G system until appropriate training has been received from a healthcare professional. Training is essential to ensure the safe use of the MiniMed™ 780G system.
WARNING: Do not use SG values to make treatment decisions, including delivering a bolus, while the pump is in Manual Mode. When the SmartGuard™ feature is active and you are no longer in Manual Mode, the pump uses an SG value, when available, to calculate a bolus amount. However, if your symptoms do not match the SG value, use a blood glucose (BG) meter to confirm the SG value. Failure to confirm glucose levels when your symptoms do not match the SG value can result in the infusion of too much or too little insulin, which may cause hypoglycemia or hyperglycemia.
Pump therapy is not recommended for people whose vision or hearing does not allow for the recognition of pump signals, alerts, or alarms. The safety of the MiniMed™ 780G system has not been studied in pregnant women, persons with type 2 diabetes using the Instinct sensor, or in persons using other anti-hyperglycemic therapies that do not include insulin. For complete details of the system, including accessories and additional important safety information concerning indications, contraindications, warnings and precautions associated with the system and its components, please consult https://www.medtronicdiabetes.com/important-safety-information and the appropriate user guide at https://www.medtronicdiabetes.com/download-library.
Important Safety Information: InPen smart insulin pen system
The InPen system is a reusable insulin pen for people living with diabetes. It can be used to deliver insulin, help calculate insulin doses, and estimate carbohydrates for meals. Those under the age of 7 should only use the device with an adult’s supervision. A healthcare provider must prescribe InPen, provide dosage settings, and discuss all potential benefits and risks. Using the device with incorrect therapy settings may lead to severe highs and lows. The InPen should not be used by those unable to test blood glucose levels or the visually impaired. For additional product and important safety information, click here.