How the MiniMed 770G system is helping one family live more and worry less
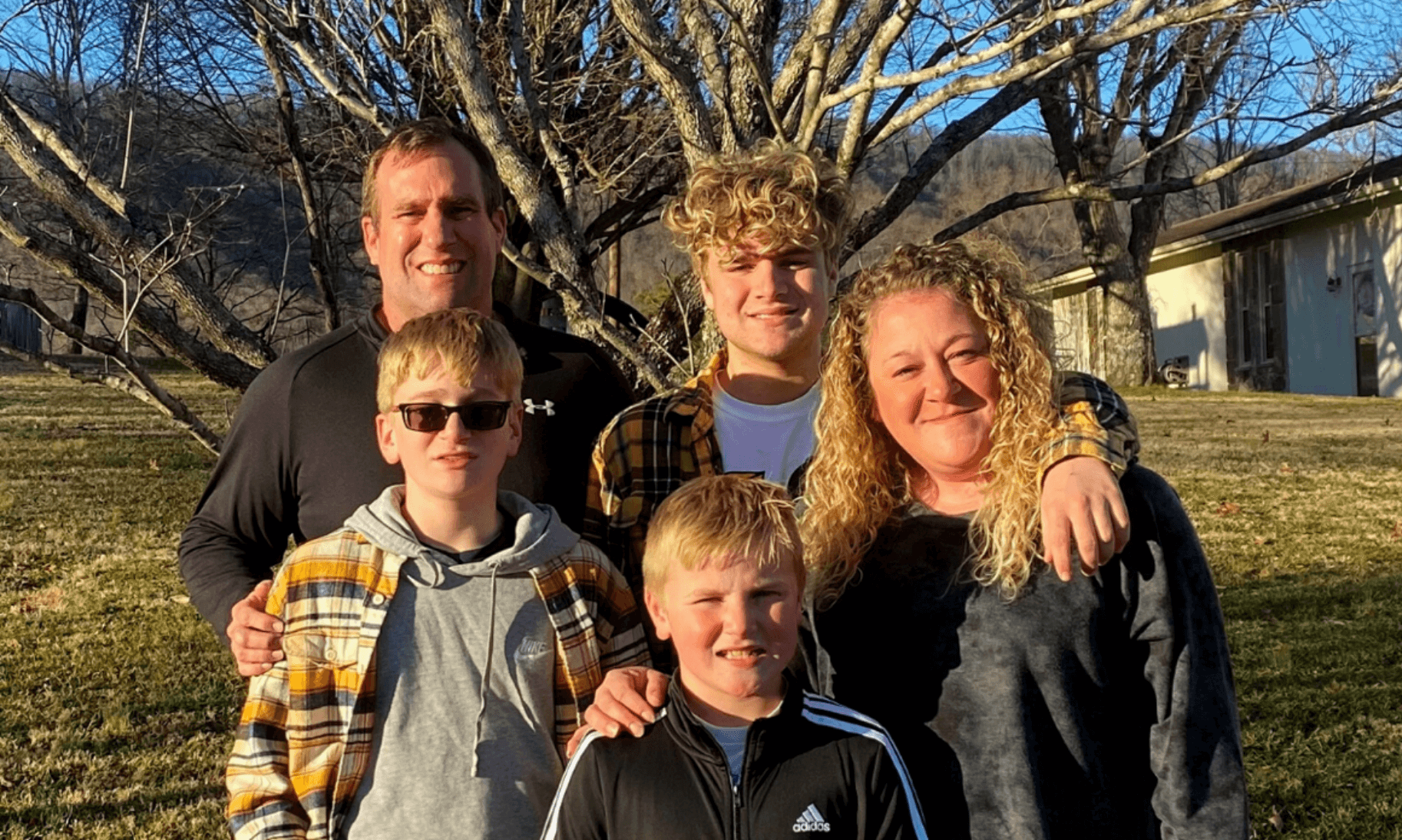
Today, we’d like to introduce you to Crystal. Crystal had two children diagnosed with diabetes within a year of each other! Today, she shares how Medtronic helped her family adjust and how the MiniMedTM 770G system is making life a little easier for them.  My husband, Tim, and I have been married for 17 years. We have 3 amazing boys together—Parker who is 15, Landyn who is 13, and Cooper who is 8. We live a very busy life full of work, church, basketball, and various other hobbies.
My husband, Tim, and I have been married for 17 years. We have 3 amazing boys together—Parker who is 15, Landyn who is 13, and Cooper who is 8. We live a very busy life full of work, church, basketball, and various other hobbies.
On May 2, 2019 our lives changed forever. I took our middle son, Landyn, to a nephrology appointment (Landyn was born with a single kidney) with some concerns. He was having some symptoms that I couldn’t figure out. I did have concerns of diabetes, as my sister-in-law also has type 1 diabetes. We were told of her underlying symptoms before her diagnosis, and those symptoms always had a place in the back of my mind. Much to our dismay, after labs and urine samples, we were sent to the emergency room with a diagnosis of type 1 diabetes.
We were terrified and worried, but the staff and doctors at Knoxville’s Children’s Hospital were not only amazing, but supportive, caring, and so helpful. We were trained the next day extensively on proper diet, needle usage, insulin types and needs, and really everything to do with diabetes. We also met the most amazing woman from Medtronic, Millie. She explained the pump process and when Landyn would be able to receive it. Six months later, he was introduced to the MiniMedTM 670G system. That was a life changer as he no longer needed four shots a day and could eat more often.
As a growing and active boy, this was a big deal. Before the pump, he would get angry, sad, and frustrated. He wanted that freedom to go to a birthday party or have that school snack without the restriction of very little to no carbs. He wanted the freedom to eat like other kids without needing to inject insulin in front of everyone. That’s why the pump was such a blessing.
Fast forward to April 19, 2020. We were officially shut down as a nation due to COVID-19. My husband continued to work while I mainly stayed home with the kids. Our oldest son, Parker, started complaining about several of the same symptoms as Landyn. He was irritable, losing weight, extremely thirsty, and urinating more frequently. We just assumed it was from being home with no schedule. He would stay up late and sleep all day if I let him. However, there was a small voice in my head that told me to check his blood sugar. it turns out he was 384 mg/dL after being asleep all night. Because we were in the middle of a pandemic, we had to be seen at the children’s primary physician to get a diagnosis. I already knew that it was type 1 diabetes but followed protocol anyway.
We were ultimately sent to Knoxville Children’s Hospital again and received the confirmation. Parker was devastated, angry, and just confused. How could he, as the older brother, be diagnosed as well? How would this affect his basketball future? Could he still play and be a part of something he had loved since he was five years old? He had so many questions that we tried to help him through. Just like Landyn, he hated the needles, he hated shots, and hated having a restricted diet. He is also a growing teenage boy. In case you hadn’t heard, they love to eat! The diagnosis also took a toll on his mental state and well being, so it was good that he had a group of friends who were there for him during this difficult time.
 My momma heart was broken. Two kids with type 1 diabetes was scary and overwhelming. I felt like we were just getting into a groove with Landyn then BAM! We had to start over and have Parker go through it all as well. Parker had seen Landyn’s struggles and was scared and obviously very stressed. Landyn was trying so hard to be comforting and supportive. But it was a struggle. Parker also had to wait the six-month period to be approved for a pump. By the time it was his turn, the MiniMedTM 770G system was out and so much more advanced.
My momma heart was broken. Two kids with type 1 diabetes was scary and overwhelming. I felt like we were just getting into a groove with Landyn then BAM! We had to start over and have Parker go through it all as well. Parker had seen Landyn’s struggles and was scared and obviously very stressed. Landyn was trying so hard to be comforting and supportive. But it was a struggle. Parker also had to wait the six-month period to be approved for a pump. By the time it was his turn, the MiniMedTM 770G system was out and so much more advanced.
He had such amazing results within the first week that our representative from Medtronic, Mille, was thrilled. It was a game changer for Parker. He was already starting to feel better and was gaining more confidence back because of the freedom a pump like this allows. He was back to playing basketball and feeing great. As for this momma, I was so thankful to see this but to also have this amazing pump with such new features. I could check everything on my phone! That was huge and such a big difference from Landyn’s MiniMedTM 670G system! I was happy to finally know exactly when he calibrated the sensor, when he would take a bolus, and how many carbs he put in. As a parent of teenagers, this was huge. They don’t always check sugar when needed or enter the correct amount of carbs. This was so helpful for me to make sure he was counting carbs correctly and doing everything he needed to. It was a huge help in holding him accountable.
Landyn also received the MiniMedTM 770G system around a month later and has been doing amazing as well. The customer and personal support from our representative was by far the most excellent care we have ever encountered. They heard of Parker’s results and made sure Landyn received the same pump to help him. I have never dealt with a company that cares for their customers as much as Medtronic. We have had to call for different reasons at different times and I can tell you we have never been disappointed. If the person on the phone couldn’t help, they would find someone who could.
I feel such a relief knowing that no matter where they are, I can follow them on the app and know what their sugars are and how much they are eating. Another function I love is it shows me the amount of insulin they have, battery life, and how many days the sensor has left. This gives them freedom to be teenagers and gives this momma peace of mind knowing my boys are safe.
As a parent, you never want your children to be sick. We have told them that diabetes is a part of your life and doesn’t define you. We encourage them to follow their dreams and never let this diagnosis hold them back. They have alerted us on the highs and lows and allows us to treat fast and efficiently. They are such a blessing. I am forever grateful for Medtronic and the support they have given my family. I know that with the support from family, friends, and insulin pumps like the MiniMedTM 770G system, our boys can lead happy and healthy lives.
The testimonial is of an individual’s experience using a Medtronic device. Talk to your doctor about your health and the risks and benefits of Medtronic devices.
IMPORTANT SAFETY INFORMATION MINIMED™ 770G SYSTEM WITH SMARTGUARD™ TECHNOLOGY
The MiniMed™ 770G system is intended for continuous delivery of basal insulin (at user selectable rates) and administration of insulin boluses (in user selectable amounts) for the management of type 1 diabetes mellitus in persons two years of age and older requiring insulin as well as for the continuous monitoring and trending of glucose levels in the fluid under the skin. The MiniMed™ 770G system includes SmartGuard™ technology, which can be programmed to automatically adjust delivery of basal insulin based on continuous glucose monitoring (CGM) sensor glucose (SG) values and can suspend delivery of insulin when the SG value falls below or is predicted to fall below predefined threshold values.
The Medtronic MiniMed™ 770G system consists of the following devices: MiniMed™ 770G insulin pump, the Guardian™ Link (3) transmitter, the Guardian™ Sensor (3), one-press serter, the Accu-Chek® Guide Link blood glucose meter, and the Accu-Chek® Guide test strips. The system requires a prescription.
The Guardian™ Sensor (3) has not been evaluated and is not intended to be used directly for making therapy adjustments, but rather to provide an indication of when a fingerstick may be required. All therapy adjustments should be based on measurements obtained using a blood glucose meter and not on values provided by the Guardian™ Sensor (3).
All therapy adjustments should be based on measurements obtained using the Accu-Chek® Guide Link blood glucose meter and not on values provided by the Guardian™ Sensor (3). Always check the pump display to ensure the glucose result shown agrees with the glucose results shown on the Accu-Chek® Guide Link blood glucose meter. Do not calibrate your CGM device or calculate a bolus using a blood glucose meter result taken from an Alternative Site. It is not recommended to calibrate your CGM device when sensor or blood glucose values are changing rapidly, e.g., following a meal or physical exercise.
WARNING: Do not use the SmartGuard™ Auto Mode for people who require less than 8 units or more than 250 units of total daily insulin per day. A total daily dose of at least 8 units, but no more than 250 units, is required to operate in SmartGuard™ Auto Mode. |
WARNING: Do not use the MiniMed™ 770G system until appropriate training has been received from a healthcare professional. Training is essential to ensure the safe use of the MiniMed™ 770G system.
Pump therapy is not recommended for people whose vision or hearing does not allow recognition of pump signals and alarms. Pump therapy is not recommended for people who are unwilling or unable to maintain contact with their healthcare professional. The safety of the MiniMed™ 770G system has not been studied in pregnant women. For complete details of the system, including product and important safety information such as indications, contraindications, warnings and precautions associated with system and its components, please consult http://www.medtronicdiabetes.com/important-safety-information#minimed-770g and the appropriate user guide at http://www.medtronicdiabetes.com/download-library.
MINIMED™ 670G SYSTEM
The Medtronic MiniMed™ 670G system is intended for continuous delivery of basal insulin (at user selectable rates) and administration of insulin boluses (in user selectable amounts) for the management of type 1 diabetes mellitus in persons, seven years of age and older, requiring insulin as well as for the continuous monitoring and trending of glucose levels in the fluid under the skin. The MiniMed™ 670G system includes SmartGuard™ technology, which can be programmed to automatically adjust delivery of basal insulin based on Continuous Glucose Monitor sensor glucose values and can suspend delivery of insulin when the sensor glucose value falls below or is predicted to fall below predefined threshold values. The system requires a prescription. The Guardian™ Sensor (3) glucose values are not intended to be used directly for making therapy adjustments, but rather to provide an indication of when a fingerstick may be required. A confirmatory finger stick test via the CONTOUR®NEXT LINK 2.4 blood glucose meter is required prior to making adjustments to diabetes therapy. All therapy adjustments should be based on measurements obtained using the CONTOUR®NEXT LINK 2.4 blood glucose meter and not on values provided by the Guardian™ Sensor (3). Always check the pump display to ensure the glucose result shown agrees with the glucose results shown on the CONTOUR®NEXT LINK 2.4 blood glucose meter. Do not calibrate your CGM device or calculate a bolus using a blood glucose meter result taken from an Alternative Site (palm) or from a control solution test. It is not recommended to calibrate your CGM device when sensor or blood glucose values are changing rapidly, e.g., following a meal or physical exercise. If a control solution test is out of range, please note that the result may be transmitted to your pump when in the “Always” send mode.
WARNING: Medtronic performed an evaluation of the MiniMed™ 670G system and determined that it may not be safe for use in children under the age of 7 because of the way that the system is designed and the daily insulin requirements. Therefore this device should not be used in anyone under the age of 7 years old. This device should also not be used in patients who require less than a total daily insulin dose of 8 units per day because the device requires a minimum of 8 units per day to operate safely. |
Pump therapy is not recommended for people whose vision or hearing does not allow recognition of pump signals and alarms. Pump therapy is not recommended for people who are unwilling or unable to maintain contact with their healthcare professional. The safety of the MiniMed™ 670G system has not been studied in pregnant women. For complete details of the system, including product and important safety information such as indications, contraindications, warnings and precautions associated with system and its components, please consult http://www.medtronicdiabetes.com/important-safety-information#minimed-670g and the appropriate user guide at http://www.medtronicdiabetes.com/download-library.