Know before your glucose goes high or low.
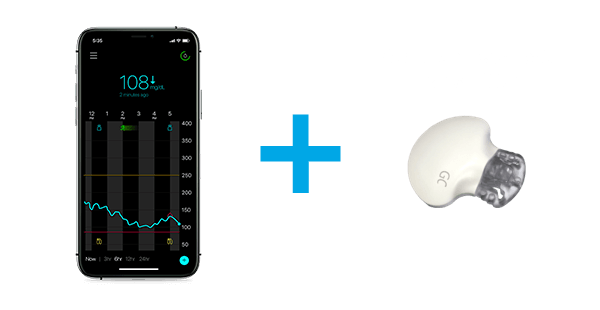
The Guardian™ Connect continuous glucose monitoring (CGM) system allows you to see glucose levels, trends and alerts on your mobile device. The system can be used by those living with type 1 or type 2 diabetes.
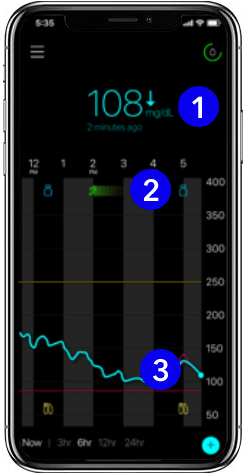
The app
1. Current glucose level
Glucose levels are updated every 5 minutes – helping you see your levels in real-time. The trend arrow next to your current reading lets you know the direction your glucose is heading.
2. Event markers
Easily track when an insulin dose is delivered, a meal is eaten, or exercise occurs. These markers will help you learn how your glucose reacts after an event.
3. Glucose trend line
See how your glucose has trended over the past 24 hours. Daily trend lines can help you understand daily patterns with glucose fluctuations.
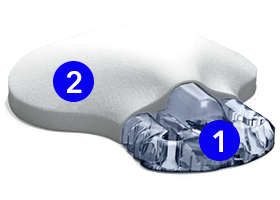
The sensor & transmitter
1. Sensor
Checks glucose levels every 5 minutes. Can be worn on the abdomen, arm, or buttock depending on your age.
2. Transmitter
Connects to the sensor and sends glucose information to your smartphone.
Stay in the know with glucose alerts.
Low glucose
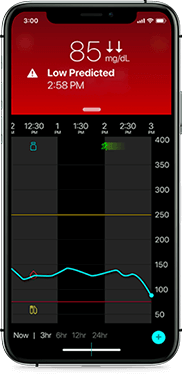
Helping you avoid lows
Receive alerts on your phone if your glucose is at or trending towards 70 mg/dL. Predictive low alerts can help you take action before your glucose dips.
High glucose
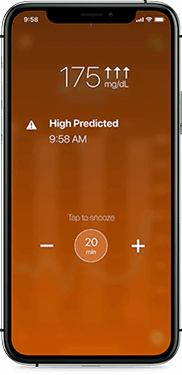
Helping you avoid highs
Receive alerts on your phone if your glucose is at or trending towards 180 mg/dL. Predictive high alerts can help you take action before your glucose rises out of range.
60-minute predictive
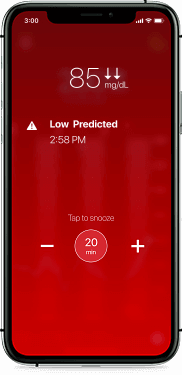
Helping you take action before your glucose is out of range
The Guardian Connect system is the only CGM that offers alerts up to 60 minutes in advance of a high or low. Predictive alerts can help you take action sooner, giving you more time to prevent your levels from going out of range.
Customizable
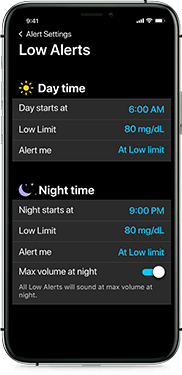
Alert settings that meet your needs
Be notified 10 to 60 minutes before your glucose is out of range. You can also adjust alert volume and mute alerts when needed. Plus, choose different settings for day and night.
Take the next step today!
Fill out the form below to get started.
We will get started on determining your insurance or pharmacy coverage. A member of our team will reach out to you for additional information. If you have questions please contact us at 888-882-8602 (M-F 9 AM-6 PM CT).

Worry less about glucose levels.
Uses InPen and Guardian Connect to help manage type 1 diabetes
Real customers. Real insights.


The support you need, when you need it
Whether you're new to injection therapy or an experienced user – our support is tailored to you. Here are the options we offer at no-cost:

24-Hour Technical Support
We offer round-the-clock support for any issues you might encounter. When you need us call 1-800-646-4633 and select option 1.

Hands-on product training
In-person or online trainings help you get started on the right foot and make sure you have everything you need to be successful on your new therapy.

Online educational resources
Interested in learning more about your diabetes device on your own? We offer a variety of resources available online to help.
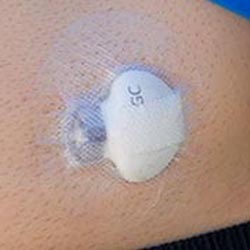
What is CGM?
Continuous glucose monitoring (CGM) systems track glucose levels, every few minutes, 24/7 through a tiny sensor inserted under your skin, either on your belly or arm using an automatic inserter. The sensor measures your interstitial glucose level, which is the glucose found in the fluid between the cells. CGM therapy can be used with or without an insulin pump.
What is smart CGM?
Smart CGM predicts future high and low sensor glucose events up to an hour in advance and provides access to information that can inform you of clinically relevant glucose patterns.†
See how it works Take the next step
Are you on multiple daily injections?
These two products might help with daily management.
InPen smart insulin pen system
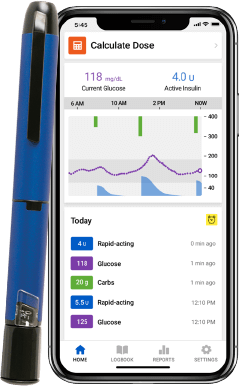
See real-time** glucose readings for your CGM in the InPen app!
bit.ly/GCRisks Ages 14-75. Rx, BG testing, proper mobile device, settings req'd. bit.ly/InPenSafety Rx, <7 requires supervision of an adult, proper settings req'd.
i-Port Advance™ injection port

Learn more about how i-Port Advance™ injection port can reduce the number of punctures from 15 to 1 over 3 days.
Frequently asked questions
The Guardian™ Connect continuous glucose monitoring (CGM) system is the standalone CGM system from Medtronic designed from the ground up to help serve the needs of customers on multiple daily insulin injections. Leveraging both modern mobile technology and the latest advances from Medtronic, the Guardian™ Connect system provides a smarter† way to manage your diabetes.
Guardian™ Connect system individuals can:
- See current glucose levels and trends, right on their mobile device, at any time
- Get alerts – including predictive alerts up to 60 minutes in advance – of high and low glucose events*
- Connect with care partners and healthcare professionals (HCPs) via the CareLink™ system platform, to enable care partner remote monitoring and HCP therapy optimization when connected to the internet via wifi/mobile data
The Guardian™ Connect system is the only CGM system with customizable predictive alerts that helps you outsmart both highs and lows. Using smart technology to predict where your glucose levels are headed, the system can alert you from 10 to 60 minutes before a high or low.
The Guardian™ Connect system compatibility information can be found by visiting Guardian™ Connect FAQs.
You should confirm compatibility with the Guardian™ Connect system using this link before updating your operating system.The transmitter used with the pump system is different and doesn’t have Bluetooth® connectivity so it isn’t compatible with the Guardian™ Connect app. The MiniMed™ 600-series pumps use the Guardian™ Link 3 transmitter, which uses a different wireless technology to communicate with the pump. The transmitter shell for the Guardian™ Connect system also has a “GC” etched on the surface opposed to the pump system transmitter shell, which just has a “G.”

User guides and manuals Training Resources
1. The system is intended to complement, not replace, information obtained from standard blood glucose monitoring devices. All therapy adjustments should be based on measurements obtained from standard blood glucose monitoring devices. All therapy adjustments should be based on measurements obtained using a home blood glucose meter and not on values provided by the system.
2. Guardian™ Connect system SSED, page 19, Table 5. 90.5% Glucose Correct Detection Rate without Predictive Alerts at both 15 and 30 minutes when worn on the abdomen.
3. Guardian™ Connect system SSED, page 19, Table 5. 98.5% Glucose Correct Detection Rate based on Predictive Alerts at 30 minutes when worn on the abdomen.
† Smart CGM predicts future high and low sensor glucose events up to 60 minutes in advance.
‡ Android is a trademark of Google LLC.
* Alerts must be set to receive predictive alerts.
** Data may not appear or be delayed in certain instances, including when there is no internet connection.
§ The patient testimonials above relates an account of an individual’s response to treatment. The account is genuine, typical and documented. However, this patient’s response does not provide any indication, guide, warranty or guarantee as to the response other people may have to the treatment. The response other individuals have to the treatment could be different. Responses to the treatment can and do vary. Not every response is the same. Please talk to your doctor about your condition and the risks and benefits of these technologies.
Important Safety Information: Guardian™ Connect CGM System
The Guardian™ Connect system requires a prescription and is indicated for continuous or periodic monitoring of glucose levels in the interstitial fluid under the skin, in patients (14 to 75 years of age) with diabetes mellitus. The system is intended to complement, not replace, information obtained from standard blood glucose monitoring devices, and is not recommended for people who are unwilling or unable to perform a minimum of two meter blood glucose tests per day, or for people who are unable or unwilling to maintain contact with their healthcare professional. The system requires a functioning mobile electronic device with correct settings. If the mobile device is not set up or used correctly, you may not receive sensor glucose information or alerts. For complete details of the system and its components, including warnings, contraindications, and precautions, please consult the user guide at http://www.medtronicdiabetes.com/support/download-library/user-guides and important safety information.
The system is intended to complement, not replace, information obtained from standard blood glucose monitoring devices. All therapy adjustments should be based on measurements obtained from standard blood glucose monitoring devices and not on values provided by the system.
Important Safety Information: CareLink™ Software
The CareLink™ software is intended for use as a tool to help manage diabetes. The purpose of the software is to take information transmitted from insulin pumps, glucose meters and continuous glucose monitoring systems, and turn it into CareLink™ reports. The reports provide information that can be used to identify trends and track daily activities—such as carbohydrates consumed, meal times, insulin delivery, and glucose readings. NOTE: CareLink™ report data is intended for use as an adjunct in the management of diabetes only and NOT intended to be relied upon by itself. Patients should consult their healthcare providers familiar with the management of diabetes prior to making changes in treatment. For more details, please consult important safety information. and the appropriate CareLink™ User Guide at http://www.medtronicdiabetes.com/support/download-library/user-guides.
Important Safety Information: i-Port Advance™ Injection Port
i-Port Advance™ injection port is indicated for patients who administer or receive multiple daily subcutaneous injections of physician prescribed medications, including insulin. The device may remain in place for up to 72 hours to accommodate multiple injections without the discomfort of additional needle sticks. i-Port Advance™ injection port may be used on a wide range of patients, including adults and children. Prescription required. For more, please see http://www.medtronicdiabetes.com/important-safety-information.
Important Safety Information: InPen™
The InPen™ is a home- use reusable pen injector for single- patient use by people with diabetes under the supervision of an adult caregiver, or by a patient age 7 and older for the self- injection of a desired dose of insulin and for calculating an insulin dose or carbohydrate intake based on user entered data. A healthcare professional must assist in dosage programming of the device prior to use, based on various patient- specific criteria and targets. The InPen™ requires a prescription. For additional product and safety information, please consult the Instructions for Use and bit.ly/InPenRisks.